specialist GMP manufacturing facilities
HERMES PHARMA has been producing medicinal products, food/dietary supplements and medical devices for more than 40 years. We have two specialist manufacturing facilities based in Germany and Austria. Our plants are equipped with very similar production technologies to ensure business continuity for most of our customers’ products, so you can rest assured that there will be no interruption to the supply of your products.
We process more than 10,300 tons of raw materials per year and generate an annual production volume of over 2.8 billion single doses:
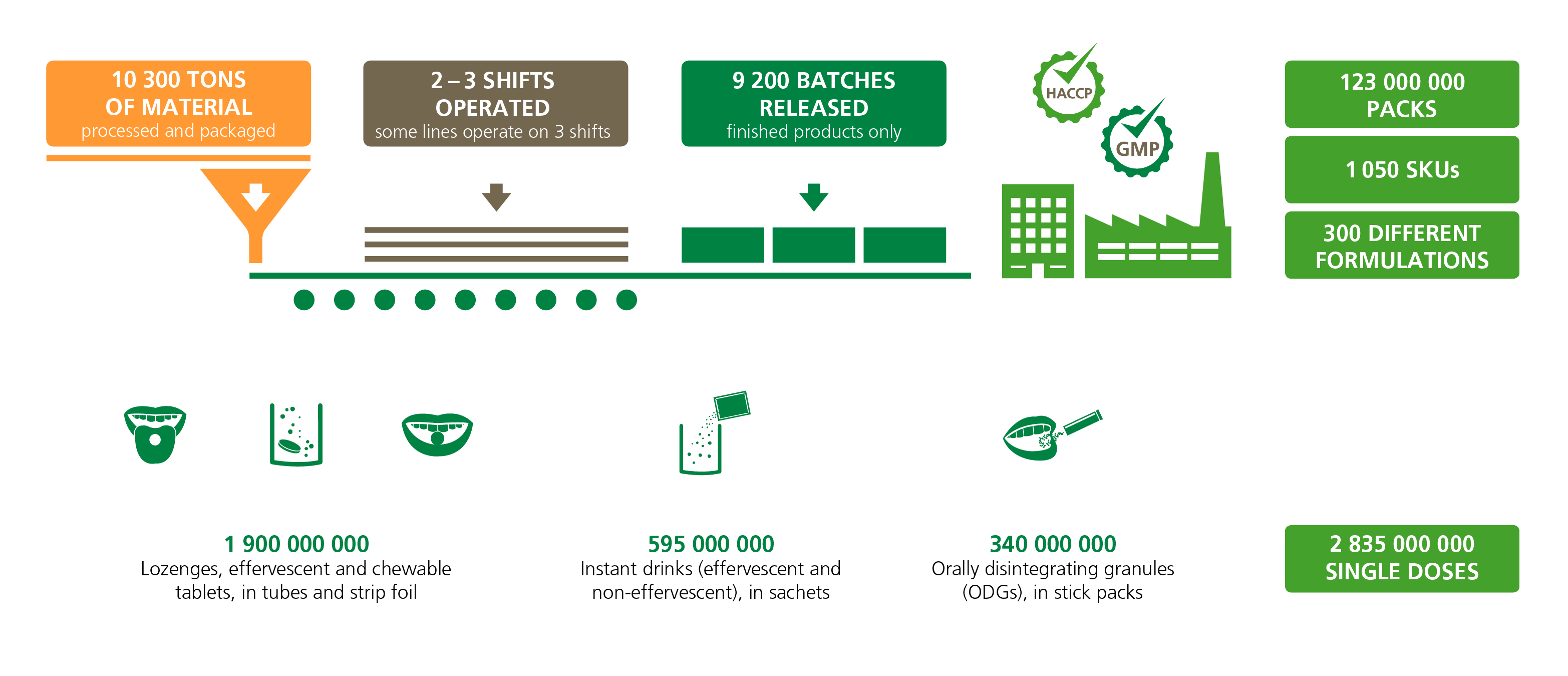
High-quality manufacturing services you can rely on
According to estimates by the WHO, up to 15% of all medicinal products worldwide are counterfeit. We believe that counterfeiting can be reduced by serializing products. The Falsified Medicines Directive 2016/161/EU (FMD) has been implemented in both of our plants to supply serialized and tamper-evident products.
Find out more about our serialization initiatives
Our plant in Wolfratshausen, Germany
Opened in 1994, our plant in Wolfratshausen is optimized for the manufacture of moisture-sensitive products, such as lozenges, chewable tablets and effervescent tablets. Weighing, blending, granulating, tableting and packaging take place in automated, closed systems spread across 12,400 square meters.
For maximum efficiency, and to reduce manufacturing time and cost, the plant was designed to minimize transportation distances for all materials at every step of the process. Rigorous quality management ensures compliance with national and international directives for the manufacture of medicinal products and food/dietary supplements and of course with our customers’ specifications.
Highlights
- Fully automated high-rack warehouse
- Automated weighing centers
- TOPO vacuum granulators
- Blenders
- Inline filling and packaging equipment
- High-speed tube filling and packaging lines with options such as attachment of fold-out labels (leporello)
- Foil strip filling lines with intelligent picking machines to place into secondary packaging
The plant in Wolfratshausen is registered / certified by various bodies (e.g. the Ministry of Upper Bavaria, the Russian Ministry of Health (MOH), the Belorussian MOH, the Food and Drug Association (FDA)), and operates according to the following standards:
- EU Good Manufacturing Practice (GMP) guidelines for medicinal products
- GMP guidelines of Russia and Belarus
- Hazard Analysis and Critical Control Point (HACCP) for the manufacture of food supplements
- US-CFR (Code of Federal Regulations) for the manufacture of food/dietary supplements intended for the US market
- ISO 50001 for sustainable energy management
HERMES PHARMA GmbH
Hans-Urmiller-Ring 52
82515 Wolfratshausen
Germany
Phone +49 8171 400 0
Fax +49 8171 400 229
Our plant in Wolfsberg, Austria
We opened this GMP facility in May 2009 in Wolfsberg, Austria. The plant consists of two main buildings and has a floor space of nearly 25,000 square meters. It encompasses 7,000 square meter cleanroom production, storage and picking areas with a fully automated high-rack warehouse, as well as laboratories and technical departments.
The plant has been specifically designed for the manufacture of user-friendly oral dosage forms, such as ODGs, instant drinks and effervescent tablets. High production flexibility and fast throughput times characterize this modern plant. Essential manufacturing steps, such as weighing raw materials and feeding continuous flow granulators from silos, are fully automated.
Highlights
- Automated weighing center
- TOPO vacuum granulators
- Continuous flow (CF) granulators
- Blenders
- Fluid bed coater
- Inline filling and packaging equipment
- High-speed sachet filling and packaging lines
- High-speed stick pack filling and packaging lines
The plant in Wolfsberg is certified by various bodies (e.g. the Austrian Agency for Health and Food Safety (AGES MEA), the Russian Ministry of Health (MOH), the Belorussian MOH and the Kazakh MOH) and operates according to the following standards:
- EU Good Manufacturing Practice (GMP) guidelines for medicinal products
- GMP guidelines of Russia, Belarus and Kazakhstan
- Hazard Analysis and Critical Control Point (HACCP) for the manufacture of food supplements
- ISO 13485 for medical devices
- Environment health and safety (EHS) for environmental protection and safety at work
HERMES PHARMA Ges.m.b.H.
Schwimmschulweg 1a
9400 Wolfsberg
Austria
Phone +43 4352 2072 0
Fax +43 4352 2072 1900

